Determining the Respirator Fit Capability of Shawmut’s Model SR9520 Respirator and Three KN95 Masks with Verified Filtration of 95% or More
The purpose of this study is to determine the range of people that properly fit Shawmut’s Model SR9520 filtering facepiece respirator (FFR) size medium/large (M/L). This information is to provide assurance that Shawmut’s Protex™ N95 Particulate Respirator, Model SR9520 will effectively fit varying face sizes and shapes. An evaluation of three KN95 masks whose filtration tested at 95% or more was conducted to compare fit performance.
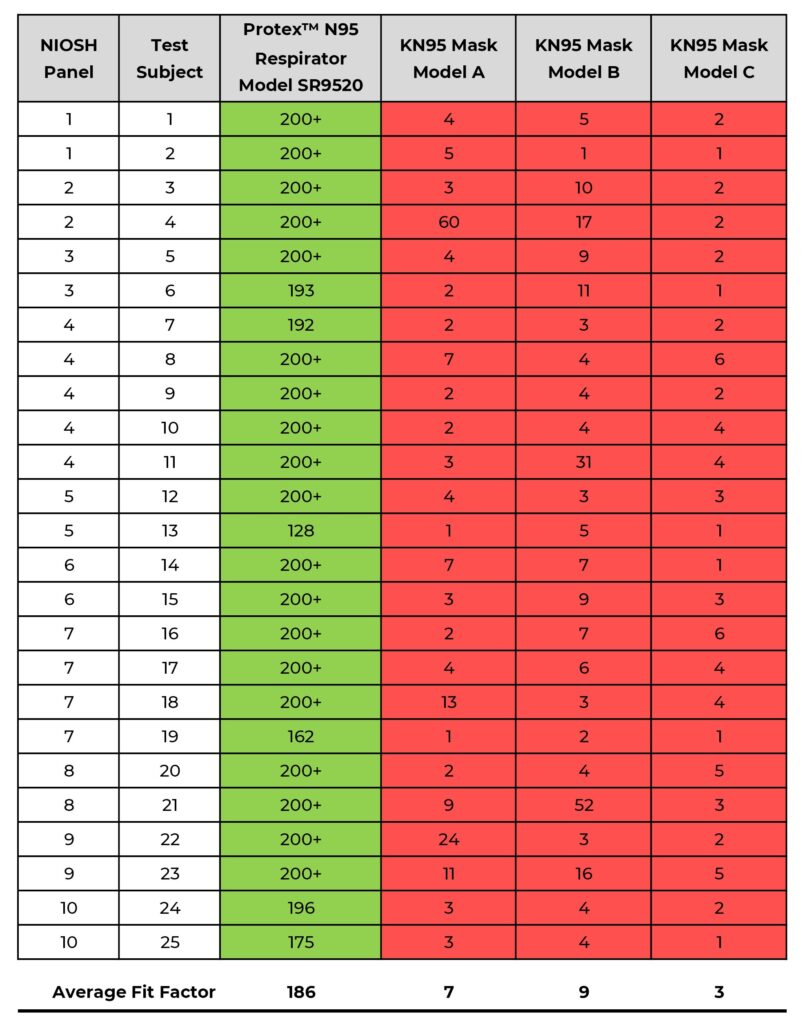
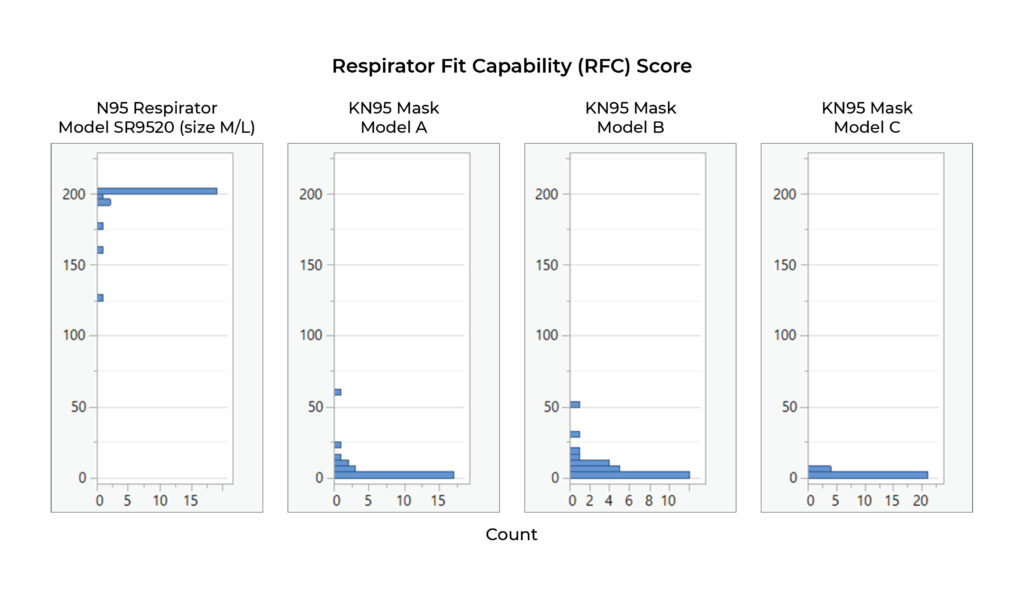
Shawmut’s Protex™ N95 Particulate Respirator, Model SR9520 (size M/L), per the requirements of ASTM F3407-20, can be expected to effectively fit a large range of people with faces of varying size and shape. The results also show that the three KN95s tested do not effectively fit any of the test subjects in the panel even though filtration efficiency was verified as being above the 95% standard. (see Table/Chart 1)
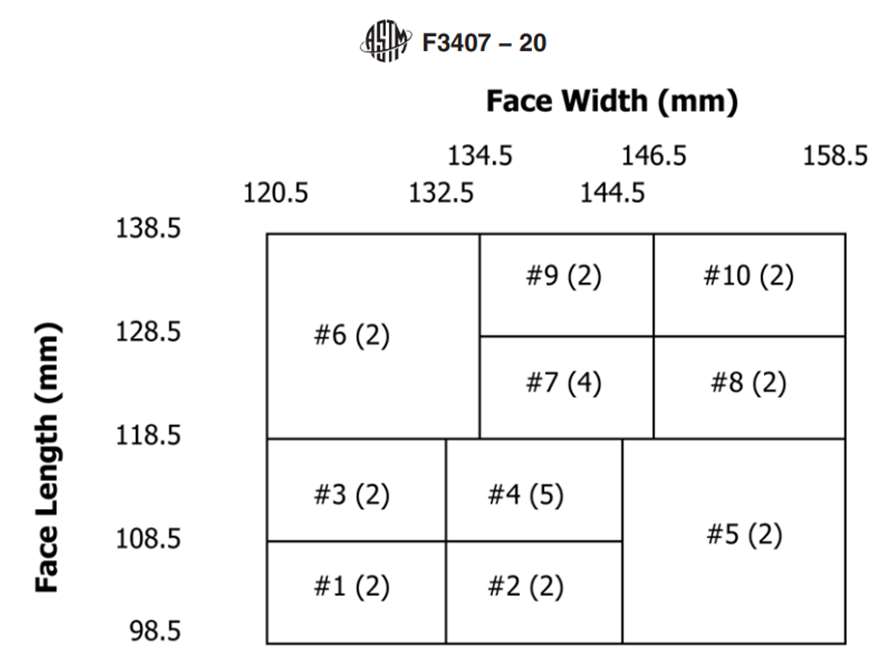
The Standard Test Method for Respirator Fit Capability for Negative-Pressure Half-Facepiece Particulate Respirators, ASTM F3407-20, provides detailed instructions for evaluating the respirator fit capability of filtering facepiece respirators. The standard uses the NIOSH Bivariate Test Panel (see Figure 1) and specifies that at least 13 of the 25 subjects (> 50%) must achieve a respirator fit capability (RFC) result of ≥ 100 for a one-size respirator.
All test equipment is calibrated and traceable to NIST standards (see Figure 2).
All test subjects were free of facial hair and other facial characteristics that would impact a proper fit. It was confirmed that all test subjects had not eaten or smoked within a half hour prior to the test. Overall, 13 males and 12 females were tested.
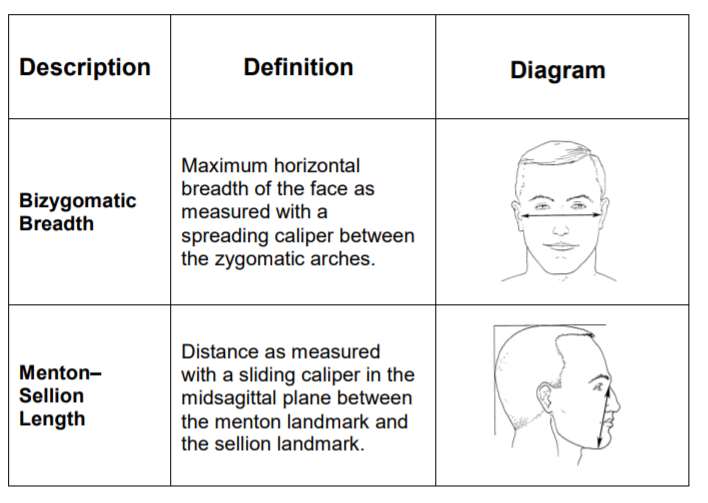
The width (zygomatic arches) and length (menton-sellion length) of the test subjects’ faces were measured (see Figure 1) using the digital caliper. The dimensions were measured twice for width, or three times if the measurement difference was greater than 2 mm. The average of the measured widths is recorded. The length of the face was measured twice, or three times if the measurement was greater than 3 mm. The average of the bizygomatic breadth and the menton-sellion length are identified with a panel number from the NIOSH panel (see Figure 1).
The masks tested were free of visual damage and randomly taken from the purchased lot of each subject model of filtering facepiece respirators (FFRs) and probed. (see Figure 3)
The filtration efficiency of each KN95 model was tested in advance. We drew three samples at random from each lot and tested them in accordance with 42 CFR 84 subpart K for N95. All samples tested above the minimum 95% standard.
Each subject was instructed how to correctly don the mask to achieve a good fit. Using a TSI PortaCount Plus® Model 8048 in the N95 companion mode, each subject was tested following the OSHA 29CFR1910.134 protocol, which consists of the following eight exercises:
All subjects were standing throughout the test. The respirator fit capability (RFC) for each exercise and the overall respirator fit capability (RFC) result are provided in the detailed output of the program.
The results of the fit testing show the following:
Manufacturers Calibration Certificate

Probed KN95 masks and N95 respirator with the probe located between the nose and mouth of the user.

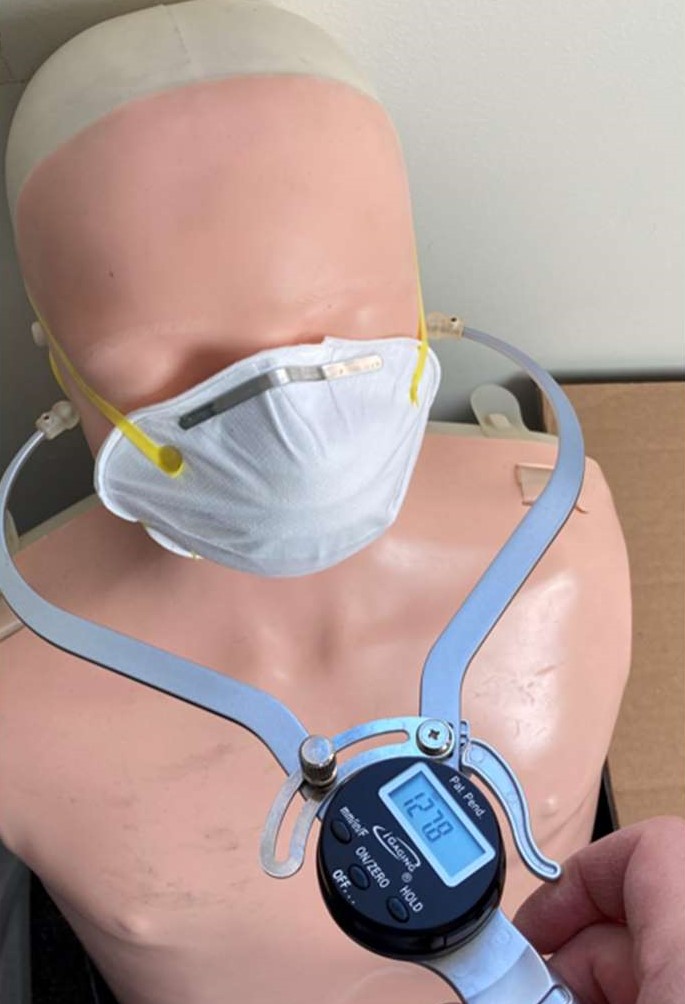
Digital caliper designed to measure face width and length.
Enclosed fit test chamber with overlapping flaps to prevent air from circulating with outside air. The chamber dimensions are 72” x 72” x 98”. RH and temperature are monitored to maintain 21-24 °C and >40%RH.


Please submit your questions and requests for information relating to this study through the contact button and our respiratory team will review them in detail.