
Are you still trusting your critical PPE to sources on the other side of the globe?
Since its founding in 1916, Shawmut Corporation has advanced materials innovation to improve people’s lives. Now, using our rapid product innovation capabilities, we have developed high-tech manufacturing operations in North America to make Protex™ Advanced Protection isolation gowns.






Non-surgical isolation gowns are a part of an overall infection-control strategy. They are a type of personal protective equipment (PPE) designed to protect the user from potentially infectious liquids and solid materials. Isolation gowns are also used to prevent cross-contamination and the transfer of microorganisms.
Isolation gowns are typically classified as disposable or reusable. Disposable, or single-use isolation gowns should be discarded after one use. Reusable isolation gowns can be worn multiple times. The manufacturer of the isolation gown should clearly define the number of washing and drying cycles that the gown was designed to withstand and a tracking system should be in place for the user to effectively track the number of washing and drying cycles.
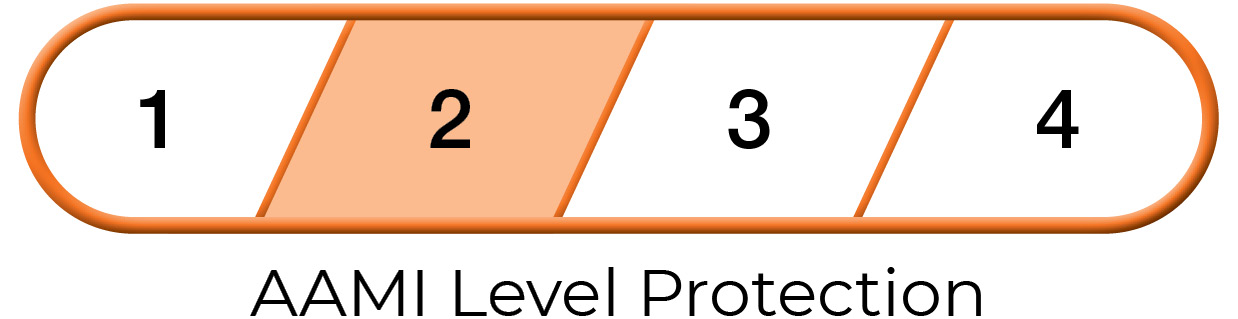
The American National Standards Institute (ANSI) and the Association of the Advancement of Medical Instrumentation (AAMI) developed standard PB70:12 to describe the barrier protection levels of gowns for use in health care settings. The standard has detailed testing methods and outlines the necessary performance results needed to verify and validate that a particular gown provides the defined levels of protection.
Contact Us Today for Made-in-the-USA Personal Protective Equipment (PPE) Solutions

Available as Berry Compliant: Meets federal regulations for Berry Amendment compliance through U.S. manufacture.
Our made-in-the-USA products are qualified for purchase under the Buy American Act.
Shawmut Corporation
Global Headquarters
208 Manley Street
West Bridgewater, MA 02379
USA
Industries Served
Capabilities
About Us
Brands
More
Legal